Spasticity After Spinal Cord Injury: When Medication Isn't the Answer
Spasticity affects the majority of people with spinal cord injury at some point in their rehabilitation journey. Estimates in reviews and educational materials suggest that roughly 65-75% of individuals will experience spasticity in the chronic stroke community phase. A large cohort study (Holtz et al. 2017) reports 65% prevalence of spasticity at community discharge in 465 patients with traumatic SCI.
The prevalence varies significantly by injury level. Spasticity is more common after cervical and thoracic injuries; one chart review found spasticity in 87% of cervical and 85% of thoracic injuries, whereas subgroups with lumbar cord or root involvement had lower rates, around 57%.”
Yet here's an important distinction: while most people develop some degree of spasticity, only about 35% experience spasticity severe enough to require treatment. Of those, 11-14% have moderate-to-severe problematic spasticity that significantly affects their daily lives.
If you're frustrated with your current spasticity management, you're not alone. In surveys, 72% of people with spasticity report it impacts their overall quality of life, 65% say it disrupts their sleep, and 44% report loss of independence (Holtz et al., 2017). Many find that anti-spasticity medications help but come with side effects that compromise quality of life in different ways. The question then becomes: what are the alternatives?
This article explores non-pharmacological approaches to spasticity management, with particular focus on electrical stimulation methods - including transcutaneous spinal cord stimulation (tSCS) - that show genuine promise in research and clinical applications.
Understanding Spasticity Development
Before discussing treatments, it helps to understand when and how spasticity develops. Research by Gao and colleagues (2024, n=175 traumatic SCI patients) has mapped the typical trajectory:
Spinal shock phase (immediately post-injury): resulting in Areflexia, flaccid muscle tone, and loss of reflexes.
Early transition (2 weeks to 4 months): This is when spasticity typically emerges. Interestingly, higher muscle tone scores at 2 weeks can predict later severity. The most significant changes occur within the first 4 months, this window may represent the optimal time for establishing management strategies.
Chronic phase (12+ months): Spasticity typically stabilises, though it can still be modified with intervention.
One noteworthy finding: in the subacute phase, incomplete injuries tend to show a higher prevalence of spasticity than complete injuries. By the chronic phase, most individuals exhibit spasticity regardless of injury completeness (Sangari & Perez, 2022).
The Medication Dilemma
The standard medications for spasticity - baclofen, tizanidine, diazepam, dantrolene - work, up to a point. These are the principal pharmacological treatments, sometimes alongside gabapentinoids and cannabinoids. They reduce muscle tone and can decrease the frequency and intensity of spasms. But the side effect profiles are concerning when you look at the research data.
Baclofen side effects (Dease et al., StatPearls):
- Overall adverse effects: 10-75% of users
- Somnolence (drowsiness): 5.7-20.9%
- Hypotonia (muscle weakness): 2-34.7%
- Dizziness: 1.7-12%
- Nausea: 1.4-10.5%
Tizanidine side effects (Ghanavatian & Derian, StatPearls):
- Drowsiness/somnolence: up to 41% in some studies
- Dry mouth: the most common complaint
- Falls: 29.2% in the FAERS database reports
- Asthenia (weakness/fatigue): significant minority
Three-quarters of patients rate common side effects as mild to moderate; one-quarter rate them as severe. Effects appear to be dose-related - the higher the dose needed for spasticity control, the more pronounced the side effects.
Drowsiness and fatigue. Many people report feeling "foggy" or constantly tired on anti-spasticity medications. This affects concentration, motivation, and the ability to engage fully with daily life.
Muscle weakness. The same mechanism that reduces spasticity can also reduce the useful muscle tone that some people rely on for transfers, sitting balance, or standing. Finding the right dose that controls spasticity without eliminating functional tone is often a delicate balance.
Cognitive effects. Some medications affect memory, concentration, and mental clarity. For people trying to work, study, or simply stay mentally sharp, this is a significant concern.
Tolerance and dependence. Over time, some people find they need increasing doses to achieve the same effect. Withdrawal from baclofen, in particular, can be serious and requires medical supervision.
None of this means you should stop taking prescribed medication without medical guidance, that would be dangerous.
Systematic reviews and NICE evidence summaries conclude that these drugs can reduce muscle tone and spasm frequency/intensity, but effect sizes are often modest and functional gains may be limited, especially at doses patients can actually tolerate. UK spinal cord injury resources (e.g., the Royal National Orthopaedic Hospital) describe oral baclofen and tizanidine as useful but often insufficient alone, noting that intrathecal baclofen is considered when oral therapy fails due to inadequate benefit or side effects.
But it does clarify why many people ask: is there another way?
When Spasticity Is Actually Useful
Before discussing alternatives, I should acknowledge something often overlooked: spasticity isn't always a problem to be solved. Some people with spinal cord injury use their spasticity functionally.
It can help with:
- Maintaining sitting posture through trunk tone
- Assisting with standing transfers
- Preserving leg muscle bulk
- Supporting circulation in the legs
If your spasticity is not causing pain, interfering with function, or leading to issues such as skin breakdown from sustained positioning, you might not need to treat it at all.
The goal of any spasticity management should be to reduce the negative effects while preserving any benefits. This is where non-pharmacological approaches have an advantage - they can often be targeted more precisely than systemic medications.
The Evidence for Electrical Stimulation
A systematic review by Bekhet and colleagues (2019) examined 23 clinical and non-clinical trials involving 389 subjects. The findings were substantial: neuromuscular electrical stimulation (NMES) and functional electrical stimulation (FES) combined produced 45-60% reductions in spasticity, alongside decreased EMG activity and increased range of motion.
A more recent systematic review and meta-analysis by Mangnall and colleagues (2022), examining 29 studies, including 5 randomised controlled trials, confirmed that electrical stimulation was effective at reducing spasticity as measured by the Modified Ashworth Scale.
Let me explain how different forms of electrical stimulation work and what the evidence shows for each.
Functional Electrical Stimulation (FES) and FES Cycling
FES applies electrical pulses to peripheral nerves or muscles to produce contractions when voluntary control is impaired, and repeated use has been shown to reduce spasticity in people with spinal cord injury and other upper motor neuron lesions. The antispastic effect is thought to arise from a combination of spinal circuit modulation and direct muscle changes rather than from a single mechanism.
The Stim2Go’s motion sensors allow any passive/active bike to support FES Cycling and much more besides
For spasticity, proposed mechanisms include:
Restoration of post‑activation depression: repeated activation of Ia afferents can partially normalise the reduced presynaptic inhibition seen in spasticity, dampening stretch reflex excitability and clonus.
Enhancement of Renshaw cell (recurrent) inhibition: antidromic volleys in motor axons may recruit Renshaw interneurons, increasing recurrent inhibition of α‑motoneurones and reducing motoneurone firing.
Induced muscle fatigue in stimulated muscles, which lowers force output and can transiently decrease resistance to passive movement and spasm intensity.
Increased reciprocal inhibition when stimulating the antagonist of a spastic muscle, suppressing overactive agonist motor pools via spinal inhibitory pathways.
In simple terms, those four points describe how FES “calms down” overactive reflexes and tight muscles.
Resetting over‑sensitive reflexes: Repeatedly activating the muscle and its nerves helps the spinal cord “re-learn” to switch reflexes off after they fire, so they do not keep triggering tightness and jerks.
Turning up built‑in brakes: FES can activate nerve cells in the spinal cord that act like internal brakes on muscle activity, so the muscles are less over‑excited.
Tiring the muscle in a useful way: When a spastic muscle is worked enough to become pleasantly tired, it cannot tighten as forcefully, so stiffness and spasms temporarily ease.
Balancing opposing muscles: Stimulating the muscle on the opposite side of a joint (for example, the front of the leg instead of the back) helps the nervous system switch off the overactive, spastic muscle, leading to smoother, more controlled movement.
What does the research show?
Multiple studies demonstrate significant reductions in Modified Ashworth Scale scores with FES Cycling. Benefits persist at 3 and 6-month follow-ups. Importantly, research from Frontiers in Physiology (2021) indicates that at least 20 FES-cycling sessions are required for significant spasticity reduction, this isn't a quick fix but requires commitment.
Alashram et al. (2022) suggested optimal parameters for FES cycling with Frequency 20-60Hz, Pulsewidth 250 microseconds, and current amplitude 10 - 140 mA. This is not too different from our practise over the last 20 years. Although we would tend to vary frequency and pulse width depending on the effect that we want to create. In all cases, we would be using biphasic rectangular pulses with a current-controlled stimulator. For most clients, we would start by suggesting 30 min of active cycling three times a week. Electrode placements would typically include, Quadriceps, Hamstrings, Glutes, Tibialis anterior and Gastrocnemius. Depending on the number of available channels, a subset of these muscles may be used in a given session.
FES Cycling delivers stimulation to the muscles in synchrony with the movement of the bike's pedals. The rhythmic movement and muscle activation seem to have a moderating effect on spasticity that extends beyond the exercise session itself, though the carry-over is typically measured in hours rather than days.
Transcutaneous Electrical Nerve Stimulation (TENS)
Strictly speaking, all the methods we are discussing are transcutaneous, but the term TENS has a specific meaning for many clinicians. TENS provides sensory-level stimulation, typically at intensities below the motor threshold, so muscles don't contract. Despite this, it can be effective for spasticity.
Research suggests high-frequency TENS inhibits microglia (inflammatory cells in the nervous system) and modulates spinal pathways through sensory input. It's a different mechanism from motor stimulation, you're essentially calming the nervous system through sensory channels.
What does the research show?
- Single sessions significantly reduce MAS scores (P < 0.01)
- Effects last **up to 4 hours**, and sometimes 24 hours - particularly for knee extensor spasticity
- Remarkably, 15 daily 15-minute TENS treatments yield comparable results to baclofen medication (Sivaramakrishnan & Solomon, 2019)
- Effective without reaching motor threshold
Optimal parameters for TENS have been suggested (Sivaramakrishnan & Solomon, 2019):
Suggested frequencies of 100 Hz with current intensity set at a sensory level (therefore below the motor threshold level). A session duration of 15 to 60 minutes, and the frequency of application should be daily for optimal results.
Electrode placement for the lower limbs could be over the hip adductors, knee extensors (particularly effective), plantar flexors, or the tibial nerve. TENS has the advantage of being simple, well-tolerated, and widely available at low cost. Its limitation is that its effects don't persist as long as those of some other approaches.
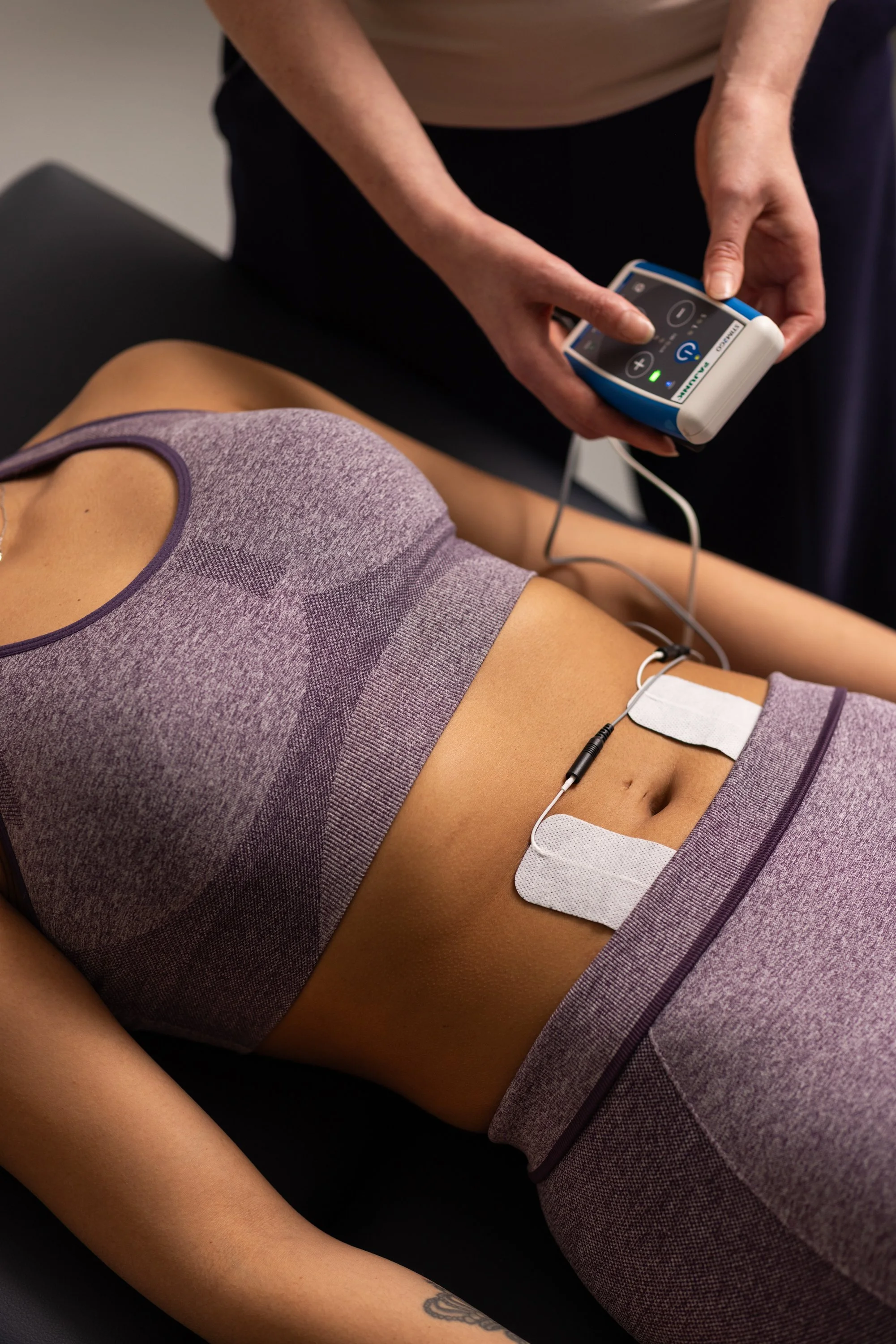
Transcutaneous Spinal Cord Stimulation (tSCS)
This is where recent developments become particularly interesting. We're starting to gain experience with this technique across a number of applications, including spasticity and pain. This is because it's one of the licensed indications of the Stim2Go stimulator, which is also the heart of our FES cycling system. In fact, as we'll discuss below, Stim2Go can combine FES cycling and transcutaneous spinal cord stimulation for spasticity in a single activity.
Transcutaneous spinal cord stimulation (tSCS) delivers electrical current to the spinal cord through electrodes placed on the skin over the spine. Unlike implanted stimulators that require surgery, tSCS is completely non-invasive - electrodes are applied before each session and removed afterwards.
How does it work for spasticity?
The mechanisms have been well characterised (Hofstoetter et al., 2019; Prem et al., 2023):
tSCS with the Stim2Go may utilise an electrode at T11/T12
- Activates afferent (sensory) fibres without requiring muscle contraction
- Transiently strengthens spinal inhibitory circuits—both pre- and postsynaptic inhibition
- Reduces tonic stretch reflexes, clonus, and cutaneous-input-evoked spasms
- Works at the source of the problem (the spinal cord) rather than affecting the whole body
While the evidence base is smaller than for peripheral stimulation methods, the results are encouraging.
Studies show reductions in both quadriceps and lower extremity muscle spasticity
- Improvements can be maintained for 10-15 days post-treatment - considerably longer than TENS or FES
- Estes et al. (2017) demonstrated improvement versus sham control
- Effects have been demonstrated across both complete and incomplete SCI, and across injury levels
Optimal parameters for tSCS
These have been suggested - Hofstoetter et al., 2019, 2024; Bilchak & Cote, 2025) - However, I don't feel that all possibilities have been explored at this point.
tSCS is performed when lying or standing
Frequencies are typically between 15 and 30 Hz, although some protocols use up to 50 Hz. The Stim2go, for example, offers 33 Hz and 50 Hz for its spasticity reduction programmes. Pulse width is typically 500 μs to 1 ms.
Intensity will typically be set at sub-motor threshold. The Stim2go has a priming programme that makes it easy to determine the intensity level. Current will vary between 20 and 100 mA and needs to be adjusted based on the individual's response. A 30-minute session duration would be typical.
For spasticity in the lower limbs, the cathode electrode would be placed paravertebrally at T11/T12 or L1/L2 intervertebral space. The anode would typically be at the bilateral iliac crests or an abdominal placement.
The Stim2Go system we provide includes approved tSCS programmes specifically for spasticity management, using these evidence-based parameters. Treatment can be done lying down or standing (e.g., in a standing frame).
Comparing Carry-Over Effects
TENS can produce a significant MAS reduction as an immediate effect, with a carryover of 4 hours (up to 24 hours for knee extensors)
FES can produce a significant MAS reduction as an immediate effect, with a 4-hour carryover, but long-term programmes demonstrate more lasting benefits.
NMES can produce a significant reduction in symptoms, with a variable carry-over duration reported of between 10 minutes and 6 hours
tSCS can produce a rapid and significant reduction in symptoms with a carryover duration of 10-15 days in some studies.
This helps explain treatment frequency recommendations: TENS benefits from daily use because effects are shorter-lived, while tSCS may require less frequent sessions once established.
Physical Approaches
Before moving to technology, it's worth noting traditional physical approaches that remain valuable:
Stretching. Regular, prolonged stretching of affected muscles can reduce spasticity, at least temporarily. Standing programmes, where the body's weight stretches ankle and leg muscles, have shown benefit.
Adjusting Positioning. Proper seating and positioning can minimise the triggers that provoke spasms in some people.
Exercise. Physical activity, including FES Cycling for those with paralysis, can help regulate muscle tone. The rhythmic movement and muscle activation appear to moderate spasticity. Of course, if you don't have a full FES cycling system, a passive-active bike can be beneficial.
Temperature. Cold application can temporarily reduce spasticity in some people, though the effect is short-lived.
These approaches work for many people as part of a daily routine, though their effects are often temporary and they require ongoing effort. In the initial use of an FES cycling system, we might encourage users to perform some passive stretching before starting stimulation. Alternatively, just get the legs moving with the bike before engaging the stimulation.
Combining Approaches
One advantage of electrical stimulation is that different methods can be combined:
tSCS combined with FES Cycling. There's theoretical and emerging practical evidence that combining spinal cord stimulation with FES Cycling may produce synergistic benefits. The tSCS primes the spinal circuits while the cycling provides repetitive, patterned muscle activation. Some of our clients use this combination, and it's one of our standard templates within the Stim2Go unit.
tSCS or FES alongside reduced medication. Given the 45-60% reduction in spasticity achievable with proper electrical stimulation protocols, some people may be able to reduce medication dosage, lessening side effects while maintaining spasticity control. This should only be done under medical supervision, with gradual tapering.
tSCS as part of a physical training programme. The temporary reduction in spasticity after a tSCS session - which can last up to 10-15 days - may create a window for more effective stretching, positioning, or physical therapy.
Practical Considerations
If you're considering electrical stimulation for spasticity management, here are the key points:
Assessment is necessary. Not everyone is suitable for tSCS or FES, and it won't help everyone who tries it. A proper evaluation of your spasticity pattern, injury level, and overall situation helps determine whether it's worth trying.
It requires consistency of application. The research suggests a minimum of 20 sessions is needed for significant, sustained benefits with FES cycling. While single tSCS sessions show effects, the best outcomes come from regular use over weeks and months.
Timing may matter. The research suggests spasticity develops most rapidly in the first 4 months after injury. While electrical stimulation can help at any stage, early intervention during this period of high neural plasticity may be optimal.
Parameters are highly important. The difference between effective and ineffective electrical stimulation often comes down to correct parameters—frequency, pulse width, intensity, and electrode placement all affect outcomes. This is why proper guidance and approved programmes are important.
It's not a replacement for all medications. Some people can reduce their medication with electrical stimulation; others use it as an add-on that improves overall control. Discuss any medication changes with your doctor.
Other Non-Pharmacological Options
Electrical stimulation isn't the only non-drug approach to spasticity. Depending on your situation, others may be relevant:
Botulinum toxin injections. For localised spasticity in specific muscles, Botox injections can be very effective. They're typically administered every few months and target particular problem areas.
Intrathecal baclofen pumps. For severe spasticity not controlled by oral medications, surgically implanted pumps deliver baclofen directly to the spinal fluid. This requires surgery and ongoing management but can be transformative for appropriate candidates as it allows the dose to be optimised.
Each of these has its place, and the right approach depends on your specific situation.
Finally
Is electrical stimulation a miracle cure for spasticity? No. Nothing is, unfortunately. But the evidence is now substantial: properly applied electrical stimulation can achieve significant reductions in spasticity, with effects lasting from hours (with TENS) to potentially weeks (with tSCS).
For many people frustrated with the side effects of medication - particularly the 10-75% who experience adverse effects from baclofen, or the 41% who experience drowsiness from tizanidine - electrical stimulation offers a genuine alternative worth considering.
The approach is non-invasive, has minimal side effects, and can be done at home once you have the equipment and training. For some, it may reduce reliance on medications; for others, it's a useful addition to existing management.
If you're struggling with spasticity and the side effects of medication, electrical stimulation - whether TENS, FES, or tSCS - is worth investigating. A proper assessment can determine which approach is likely to help in your case, what parameters would be appropriate, and how to integrate it into your existing management.
We're happy to discuss whether electrical stimulation might be appropriate for your situation. Contact us to discuss your spasticity management and the options that might help.
References
Systematic Reviews and Meta-Analyses
Bekhet AH, Bochkezanian V, Saab IM, Gorgey AS. The Effects of Electrical Stimulation Parameters in Managing Spasticity After Spinal Cord Injury: A Systematic Review. American Journal of Physical Medicine & Rehabilitation. 2019 Jun;98(6):484-499. doi: 10.1097/PHM.0000000000001064. PMID: 30300228.
Mangnall E, Wilmshurst S, et al. Neurophysiological and Clinical Outcome Measures of the Impact of Non-Invasive Electrical Stimulation on Spasticity in Post-Stroke and Spinal Cord Injury Patients: A Systematic Review and Meta-Analysis. Frontiers in Rehabilitation Sciences. 2022;3:1047750. doi: 10.3389/fresc.2022.1047750. PMID: 36589715; PMCID: PMC9801305.
Hook MA, Woller SA, Bhatt E, et al. Evidence of Treating Spasticity Before it Develops: A Systematic Review of Spasticity Outcomes in Acute Spinal Cord Injury Interventional Trials. Therapeutic Advances in Neurological Disorders. 2022;15:17562864221088582. doi: 10.1177/17562864221088582. PMID: 35592593; PMCID: PMC8859674.
Alashram AR, Annino G, Padua E, Romagnoli C, Mercuri NB. Changes in Spasticity Following Functional Electrical Stimulation Cycling in Patients with Spinal Cord Injury: A Systematic Review. Journal of Exercise Rehabilitation. 2022;18(1):47-54. doi: 10.12965/jer.2142724.362. PMID: 35356117; PMCID: PMC8890523.
Prem V, Ragupathy R, Purwar N, et al. Transcutaneous Spinal Cord Stimulation Effects on Spasticity in Patients with Spinal Cord Injury: A Systematic Review. Journal of Spinal Cord Medicine. 2023 Jul;46(4):582-589. doi: 10.1080/10790268.2021.1994137. PMID: 34855565; PMCID: PMC10274551.
Original Research Studies
Gao Y, Kadowaki T, Nakae Y, et al. Predicting the Progression of Spasticity in the Early Phase of Spinal Cord Injury: A Prospective Cohort Study. Journal of Neurotrauma. 2024;41(9-10):1063-1071. doi: 10.1089/neu.2023.0191. PMID: 37772699.
Sangari S, Perez MA. Prevalence of Spasticity in Humans with Spinal Cord Injury with Different Injury Severity. Journal of Neurophysiology. 2022;128(3):470-479. doi: 10.1152/jn.00089.2022. PMID: 35507475; PMCID: PMC9423778.
Sivaramakrishnan A, Solomon JM. Comparison of Transcutaneous Electrical Nerve Stimulation (TENS) and Functional Electrical Stimulation (FES) on Spasticity in Spinal Cord Injury - A Pilot Randomised Double Blind Trial. Journal of Spinal Cord Medicine. 2019;42(2):181-187. doi: 10.1080/10790268.2018.1450442. PMID: 29067867; PMCID: PMC6055976.
Hofstoetter US, Krenn M, Danner SM, et al. Transcutaneous Spinal Cord Stimulation Induces Temporary Attenuation of Spasticity in Individuals with Spinal Cord Injury. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2019;27(9):1838-1847. doi: 10.1109/TNSRE.2019.2933509. PMID: 31333064.
Hofstoetter US, Freundl B, Lackner P, Binder H, Minassian K. Transcutaneous Spinal Cord Stimulation Neuromodulates Pre- and Postsynaptic Inhibition in the Control of Spinal Spasticity. Cell Reports Medicine. 2024;5(11):101805. doi: 10.1016/j.xcrm.2024.101805. PMID: 39532101; PMCID: PMC11604492.
Bilchak JN, Cote MP. Transcutaneous Spinal Cord Stimulation at Alternating Intensities Improves Spasticity After Spinal Cord Injury by Restoring Chloride Homeostasis and Spinal Inhibition. Journal of Physiology. 2025 Sep 1;603(17):4845-4865. doi: 10.1113/JP287100. PMID: 40785043; PMCID: PMC12400786.
Estes SP, Iddings JA, Field-Fote EC. Priming Neural Circuits to Modulate Spinal Reflex Excitability. Frontiers in Neurology. 2017 Feb 2;8:17. doi: 10.3389/fneur.2017.00017. PMID: 28217104; PMCID: PMC5289982.
Holtz KA, Lipson R, Noonan VK, Kwon BK, Mills PB. Prevalence and Effect of Problematic Spasticity After Traumatic Spinal Cord Injury. Archives of Physical Medicine and Rehabilitation. 2017 Jun;98(6):1132-1138. doi: 10.1016/ japmr.2016.09.124. PMID: 27780743.
Clinical Reference Resources
Dease NM, Kershner EK, Wills BK. Baclofen Toxicity. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. PMID: 35593857. Available from: https://www.ncbi.nlm.nih.gov/books/NBK580550/
Ghanavatian S, Derian A. Tizanidine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. PMID: 30137790. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519505/