How Muscle Fibre Composition Impacts Electrical Stimulation Effectiveness
Introduction
Electrical stimulation is a popular and widely used approach for physical rehabilitation. When used for muscle strengthening, it is necessary to take into account the fact that electrical stimulation produces muscle contractions in a relatively inefficient way.
In this article, we take a brief look at muscle fibre types. Following a neurological insult, muscle fibre types undergo transformation, producing changes that affect the ability of muscles to do work. We examine the particular case of spinal cord injury and consider how this affects the effectiveness of electrical stimulation.
Approaches to combat fatigue are examined. Finally, we consider attempts being made to improve the performance of stimulators and produce consistent muscle contractions under all circumstances.
Fundamentals of Muscle Fibre Types
Human skeletal muscles contain three primary fibre types, each with distinct characteristics that influence their response to electrical stimulation:
Type I (Slow-Twitch/Slow Oxidative) fibres are highly fatigue-resistant, utilising aerobic metabolism with abundant mitochondria and myoglobin. They appear red in colour, contract slowly but can sustain activity for extended periods, and are preferentially recruited for endurance activities and postural control. These fibres contain low levels of myosin ATPase activity, high oxidative capacity, and excellent capillary supply.
Type IIa (Fast Oxidative) fibres represent an intermediate category, utilising both aerobic and anaerobic pathways. They produce moderate force with good fatigue resistance, appearing pink in colour due to moderate myoglobin content. These fibres can sustain contractions for prolonged periods while generating more force than Type I fibres.
Type IIx (Fast Glycolytic) fibres rely primarily on anaerobic metabolism, producing high force rapidly but fatiguing quickly. They appear white due to low myoglobin content, have fewer mitochondria and capillaries, but contain substantial glycogen stores for rapid energy release. These fibres are recruited for explosive, high-intensity activities.
The distribution of fibre types varies between muscles and individuals, with postural muscles like the soleus containing predominantly Type I fibres, while muscles involved in rapid movements contain higher proportions of Type II fibres. Hybrid fibres exist. Many fibres co-express multiple myosin heavy chain isoforms (e.g., I/IIa, IIa/IIx) during transitions, detraining, or after neurological injury. These aren’t separate “types,” but intermediate phenotypes reflecting plasticity.
Basically, neurological conditions such as stroke, MS and Parkinson's Disease all result in muscle fibre type changes. These changes vary with the condition and the consequence is that any NMES and exercise should reflect this if possible. In this article we focus on spinal cord injury.
Spinal Cord Injury: Muscle Transformations
Following spinal cord injury, paralysed muscles undergo progressive changes that dramatically alter their fibre composition and function. This transformation occurs in distinct phases with significant implications for rehabilitation strategies.
Immediate to Early Changes (0-20 months post-injury): Initially, muscle fibre composition remains relatively stable for the first month after injury. However, between 1-20 months post-SCI, a transitional period begins, characterised by a progressive decline in Type I (slow) muscle fibres and an increase in fibres co-expressing both fast and slow myosin heavy chain isoforms.
This represents the beginning of a fundamental shift in muscle phenotype.
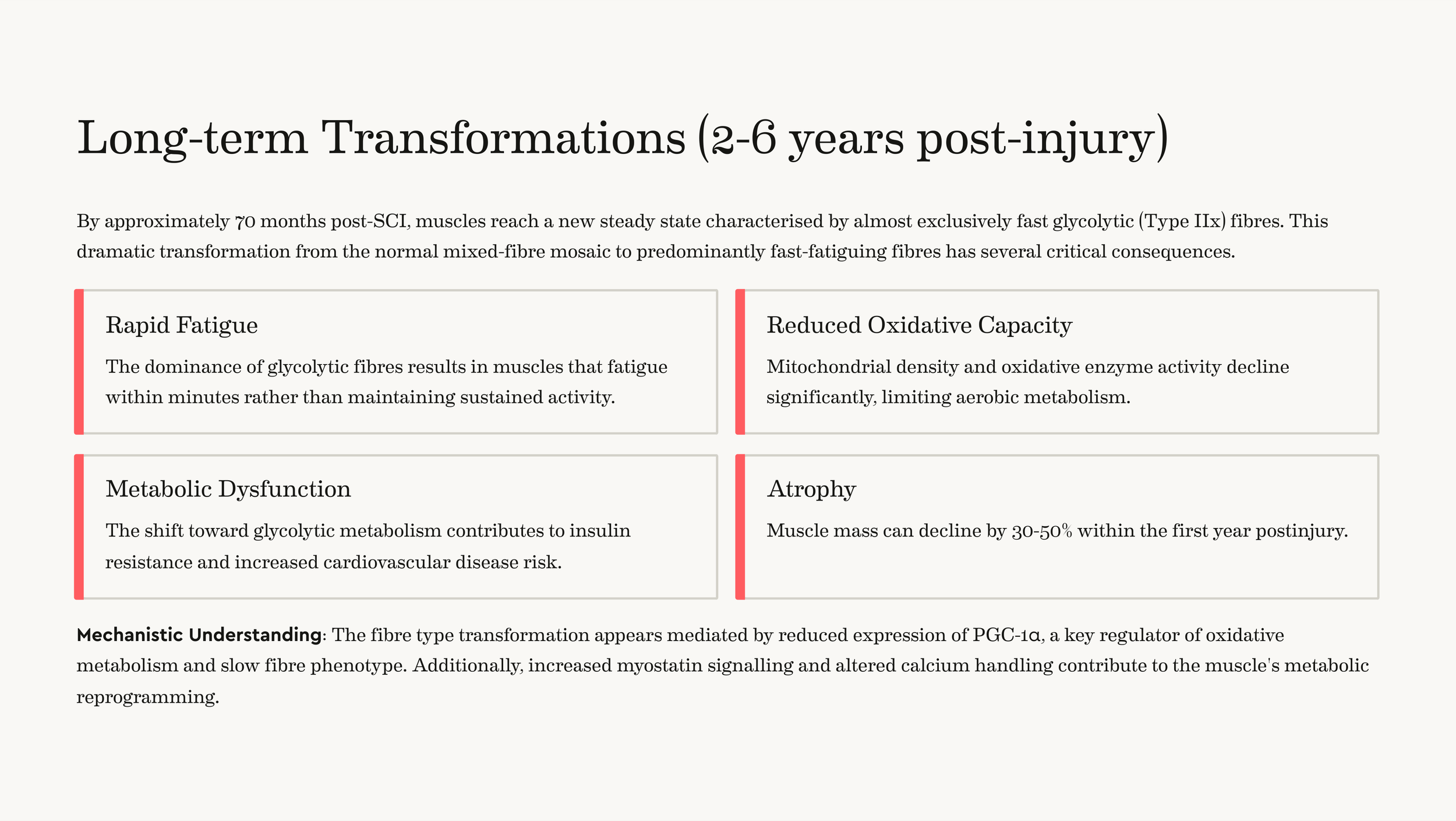
Long-term Transformations (2-6 years post-injury): By approximately 70 months post-SCI, muscles reach a new steady state characterised by almost exclusively fast glycolytic (Type IIx) fibres. This dramatic transformation from the normal mixed-fibre mosaic to predominantly fast-fatiguing fibres has several critical consequences.
Rapid Fatigue: The dominance of glycolytic fibres results in muscles that fatigue within minutes rather than maintaining sustained activity.
Reduced Oxidative Capacity: Mitochondrial density and oxidative enzyme activity decline significantly, limiting aerobic metabolism.
Metabolic Dysfunction: The shift toward glycolytic metabolism contributes to insulin resistance and increased cardiovascular disease risk.
Atrophy: Muscle mass can decline by 30-50% within the first year post-injury.
Mechanistic Understanding: The fibre type transformation appears mediated by reduced expression of PGC-1α, a key regulator of oxidative metabolism and slow fibre phenotype. Additionally, increased myostatin signalling and altered calcium handling contribute to the muscle's metabolic reprogramming.
Putting it together: The muscle’s “endurance switch” (PGC-1α) turns down, the “growth brake” (myostatin) presses harder, and the contraction control system (calcium handling) changes—together pushing muscles to become less endurance-focused and more fast, fatigable in character.
Electrical Stimulation and Muscle fibre Recruitment
Understanding how electrical stimulation recruits different fibre types is crucial for optimising rehabilitation protocols after neurological injury.
Non-Physiological Recruitment Pattern: Unlike voluntary contractions that follow Henneman's size principle (small, fatigue-resistant fibres are recruited first), electrical stimulation recruits muscle fibres based on their proximity to surface electrodes. This results in:
Random recruitment of Type I and Type II fibres regardless of force requirements.
Synchronous activation of all recruited fibres with each stimulus pulse.
Spatially fixed patterns that cannot adapt during contractions.
Implications for Fatigue: This non-physiological recruitment contributes to rapid fatigue development because:
Large, easily-fatigable Type II fibres are recruited at low stimulation intensities.
Synchronous activation prevents the natural alternation between motor units.
The inability to redistribute work among different fibres accelerates metabolic stress.
Parameter Optimisation for Fibre Type Targeting:
Recent research has identified stimulation parameters that can preferentially target different pathways:
Wide pulse widths (500-1000 μs) preferentially activate sensory fibres, potentially recruiting fatigue-resistant motor units through spinal reflexes. Most commonly available NMES units will deliver biphasic rectangular pulses although not all will be capable of delivering such wide pulses. Basically, longer pulse widths deliver more electrical charge at a given amplitude level. Sensory (afferent) nerves, especially large-diameter Ia afferents from muscle spindles, have lower activation thresholds to long-duration pulses compared with many motor axons at the same amplitude. So with wide pulses, you often excite these sensory fibres first. Electrode placement, ramp times, and task context (e.g., weight-bearing) influence how much Ia-mediated fibre recruitment you get.
Lower frequencies (20-30 Hz) may be more tolerable and effective for sustained contractions compared to higher frequencies.
Optimised pulse charge (product of frequency and pulse width) can maximise force production with reduced fatigue.
Practical implications
If your goal is endurance/activation with less fatigue: Consider wider pulse widths (500–800+ μs), moderate frequencies (20–35 Hz), longer on-times and higher duty ratios (e.g., 1:5–1:7) to leverage reflex-mediated, orderly recruitment. Very wide pulses may be uncomfortable so the amplitude level will need to be monitored carefully
If your goal is power/strength: You may accept narrower pulse widths with higher frequencies (35–50 Hz) and shorter duty cycles (e.g., 1:3–1:5), knowing recruitment will skew less toward fatigue-resistant units.
Comfort and safety: Wider pulses can feel stronger at the same current because charge is higher; adjust amplitude carefully and monitor skin and patient tolerance.
Advanced Fatigue Reduction Techniques
Several innovative approaches have emerged to minimise muscle fatigue during electrical stimulation in neurological rehabilitation:
1) Spatially Distributed Sequential Stimulation (SDSS)
SDSS represents an advancement in reducing fatigue by distributing stimulation across multiple electrode sites:
Mechanism: Instead of using a single electrode pair, SDSS employs multiple active electrodes that deliver stimulation sequentially with phase shifts between electrodes. For example, four electrodes might deliver pulses with 90° phase shifts, effectively reducing the stimulation frequency per electrode from 40Hz to 10Hz while maintaining overall muscle activation at 40Hz.
Benefits:
Reduces muscle fatigue by 30-50% compared to conventional stimulation.
Allows different muscle regions to recover between stimulations
Maintains physiological firing frequencies for individual muscle compartments.
Produces higher sustained power output during functional tasks.
Clinical Evidence: Studies in both able-bodied individuals and those with spinal cord injury have demonstrated that SDSS maintains force production significantly longer than conventional single-electrode stimulation, with some studies showing doubled endurance capacity.
2) Interleaved Stimulation Protocols
Muscle-Nerve Interleaving: This technique alternates stimulation between muscle belly and nerve trunk locations, recruiting different motor unit populations with each stimulus. By reducing motor unit discharge rates by half while maintaining overall contraction strength, fatigue can be reduced by 30-40%.
Multi-muscle Interleaving: Alternating stimulation between synergistic muscle groups allows individual muscles to recover while maintaining functional movement patterns.
3) Closed-Loop Adaptive Control Systems
Advanced control systems potentially represent the cutting edge of fatigue management:
Real-time Parameter Adjustment: Systems that monitor muscle response through EMG or force feedback and automatically adjust stimulation parameters to maintain target performance while minimising fatigue. These systems can:
Detect early fatigue indicators and modify stimulation intensity
Adjust stimulation timing based on individual muscle response patterns
Optimise electrode selection in multi-channel systems
Machine Learning Integration: AI-powered systems can learn individual response patterns and predict optimal stimulation parameters for sustained performance. Recent developments include neural networks that can predict muscle fatigue onset and automatically adjust protocols accordingly.
4) Vibration-Enhanced Stimulation
Tendon Vibration Superimposition: Applying mechanical vibration (typically 50-70 Hz) to tendons during electrical stimulation can enhance motor unit recruitment through spinal reflex pathways. While results show individual variability, approximately 50% of users experience:
Increased total muscle work capacity
Reduced voluntary muscle fatigue post-stimulation
Enhanced recruitment of fatigue-resistant motor units.
Mechanisms: Tendon vibration activates Ia afferent fibres, potentially triggering persistent inward currents at the motor neuron level and promoting more physiological recruitment patterns.
Optimised Stimulation Parameters
Pulse Width Optimisation: Research consistently shows that longer pulse widths (500-1000 μs) compared to conventional narrow pulses (100-200 μs) can:
Preferentially activate sensory pathways leading to more physiological recruitment
Increase the likelihood of recruiting fatigue-resistant motor units through spinal reflexes
Reduce the current intensity required for given force levels, improving comfort
Frequency Optimisation:
Lower frequencies (20-30 Hz) generally produce less fatigue than higher frequencies (50-100 Hz)
Variable frequency protocols that dynamically adjust stimulation frequency based on muscle state can extend endurance.
Doublet frequency trains at 20 Hz may be superior to constant frequency trains for individuals with SCI.
Combined Intervention Approaches
Blood Flow Restriction (BFR) + Electrical Stimulation: Combining mild blood flow restriction with electrical stimulation can enhance training adaptations while using lower stimulation intensities, potentially reducing fatigue.
High-Intensity Interval Training (HIIT) Protocols: Structured rest periods between high-intensity stimulation bouts allow muscle recovery and can extend overall training duration.
Hybrid Systems: Combining electrical stimulation with mechanical assistance (motorised devices) can maintain functional movement patterns while managing muscle fatigue.
Clinical Implementation Considerations
Individual Optimisation: The high variability in individual responses to different fatigue reduction techniques necessitates personalised approaches. Approximately 50% of individuals may respond positively to techniques like vibration enhancement, while others may benefit more from alternative strategies.
Progressive Training Protocols: Starting with shorter stimulation sessions and gradually increasing duration allows muscles to adapt and build endurance capacity over time.
Multi-Modal Monitoring: Effective fatigue management requires monitoring multiple parameters including:
EMG amplitude and frequency changes
Force production patterns
Subjective comfort levels
Metabolic indicators where available
The field of electrical stimulation for neurological rehabilitation continues to evolve rapidly, with emerging technologies like optogenetic stimulation showing promise for even more physiological muscle control.
However, current techniques, when properly applied and individually optimised, can significantly improve the effectiveness of electrical stimulation therapy by managing muscle fatigue and promoting more sustainable training adaptations.
In summary, understanding the fundamental characteristics of muscle fibre types and the profound transformations that occur following spinal cord injury is essential for shaping effective rehabilitation strategies. As fast-fatiguing fibres become predominant after injury, electrical stimulation must be carefully optimised—through pulse width, frequency, sequencing, and advanced technologies—to address rapid fatigue and promote functional gains. By applying evidence-based stimulation protocols and continually monitoring outcomes, practitioners can better target the unique needs of each individual, ultimately enhancing neuromuscular recovery and maximising training potential in neurorehabilitation.
For the best outcomes we prefer to use devices that allow fine control of all aspects of stimulation. Many devices on the market restrict access to stimulation parameters. All of our systems, such as the Stim2Go, KT Motion range, the RISE Stimulator or Edition 5 offer best in class performance and of course we aim to deliver best of class support to our clients.
Related Article
Zone 2 Training for Spinal Cord Injuries?Unlocking the Benefits of Low-Intensity Cardio for Health and Performance